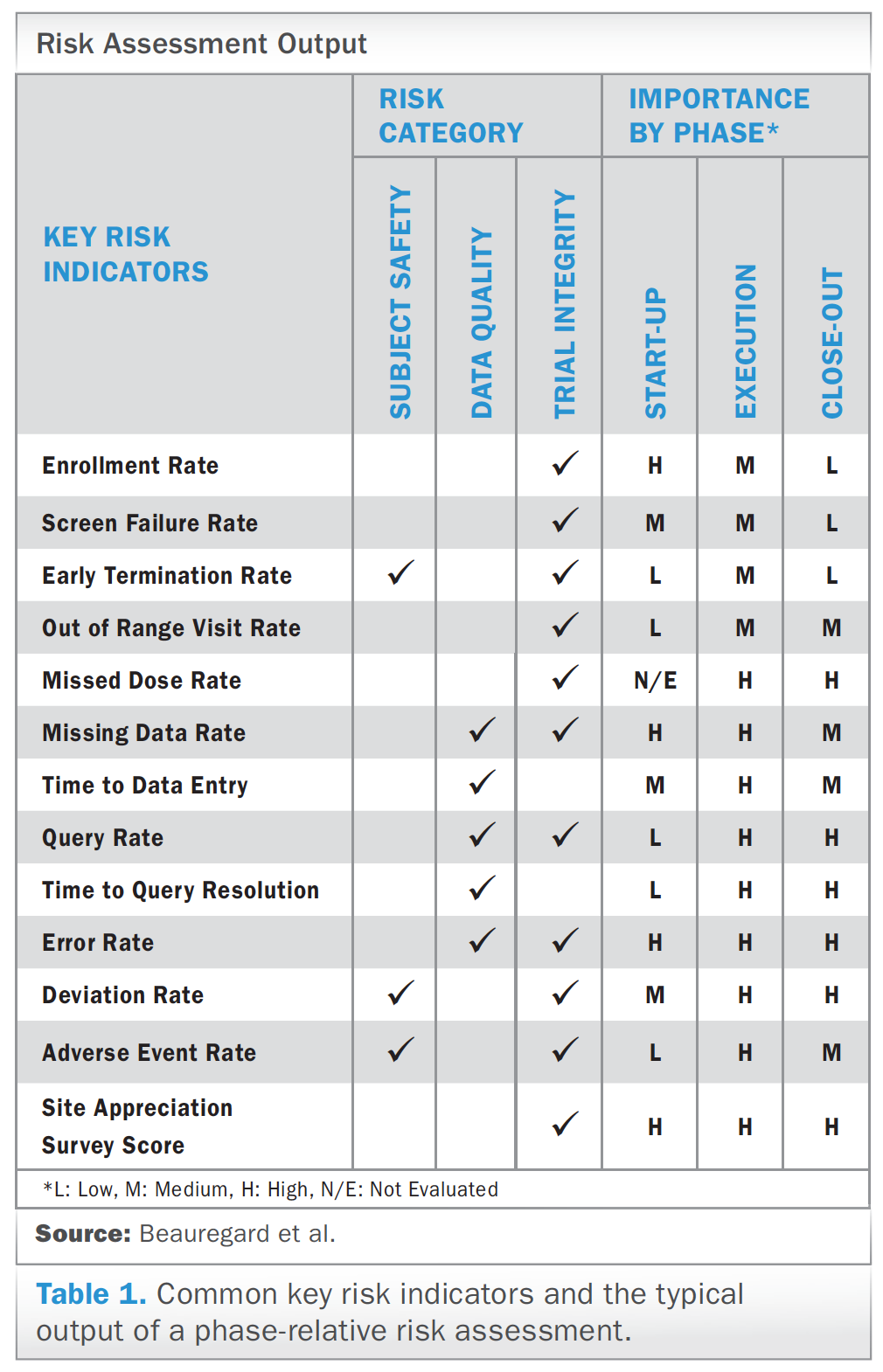
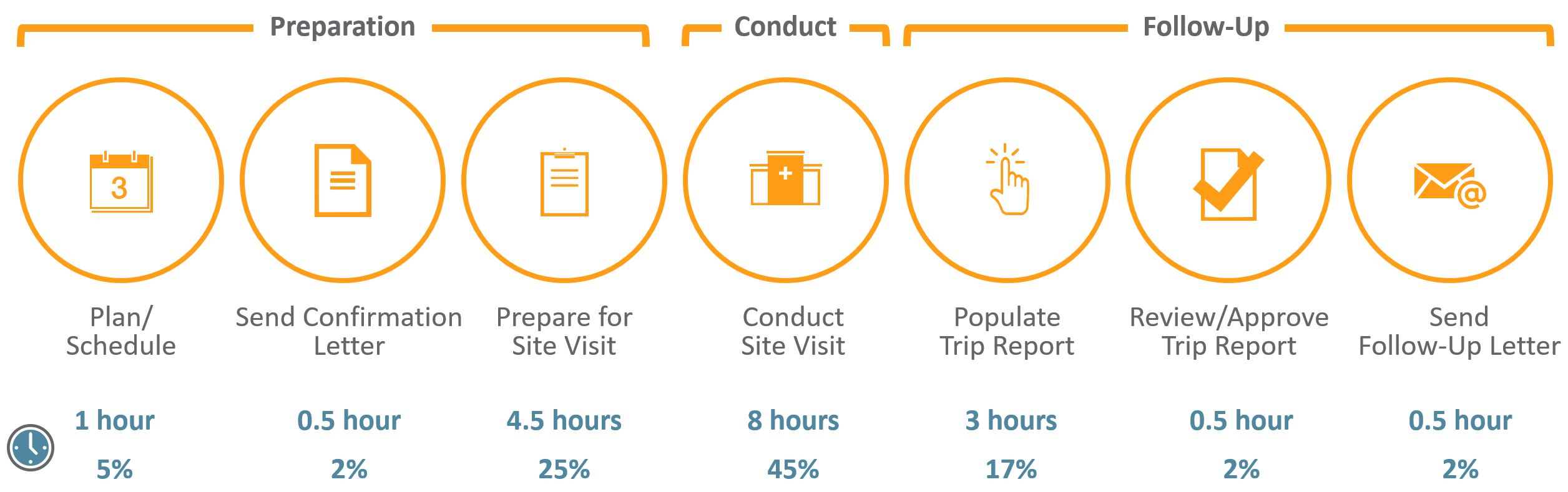
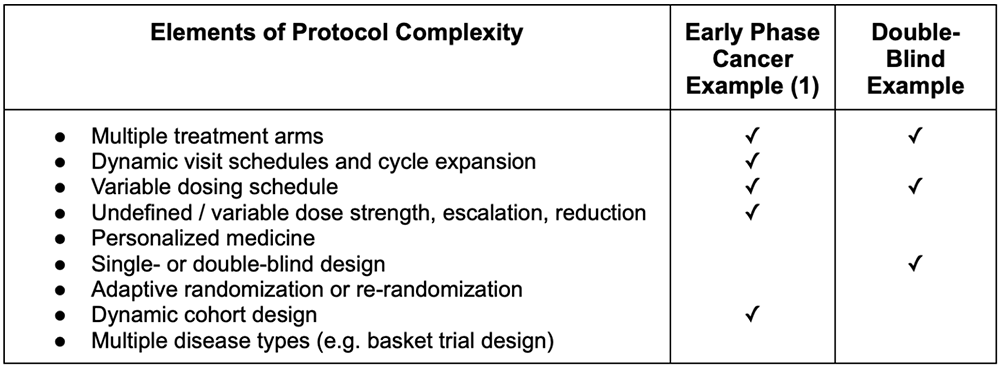
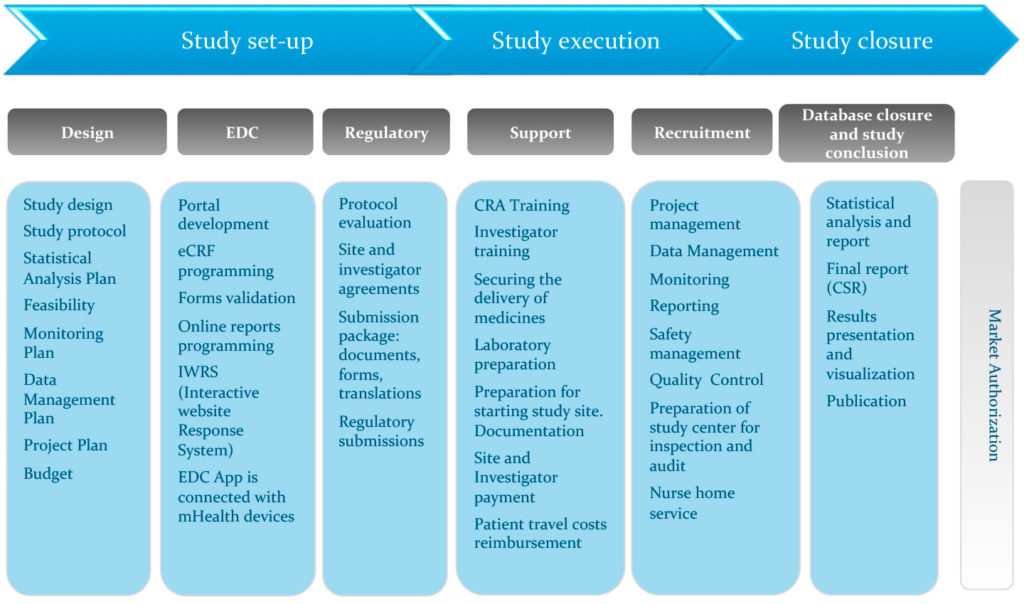
David Cloutier Director, Research Center Management and Development Budgeting for Industry Sponsored Clinical Trials. - ppt download

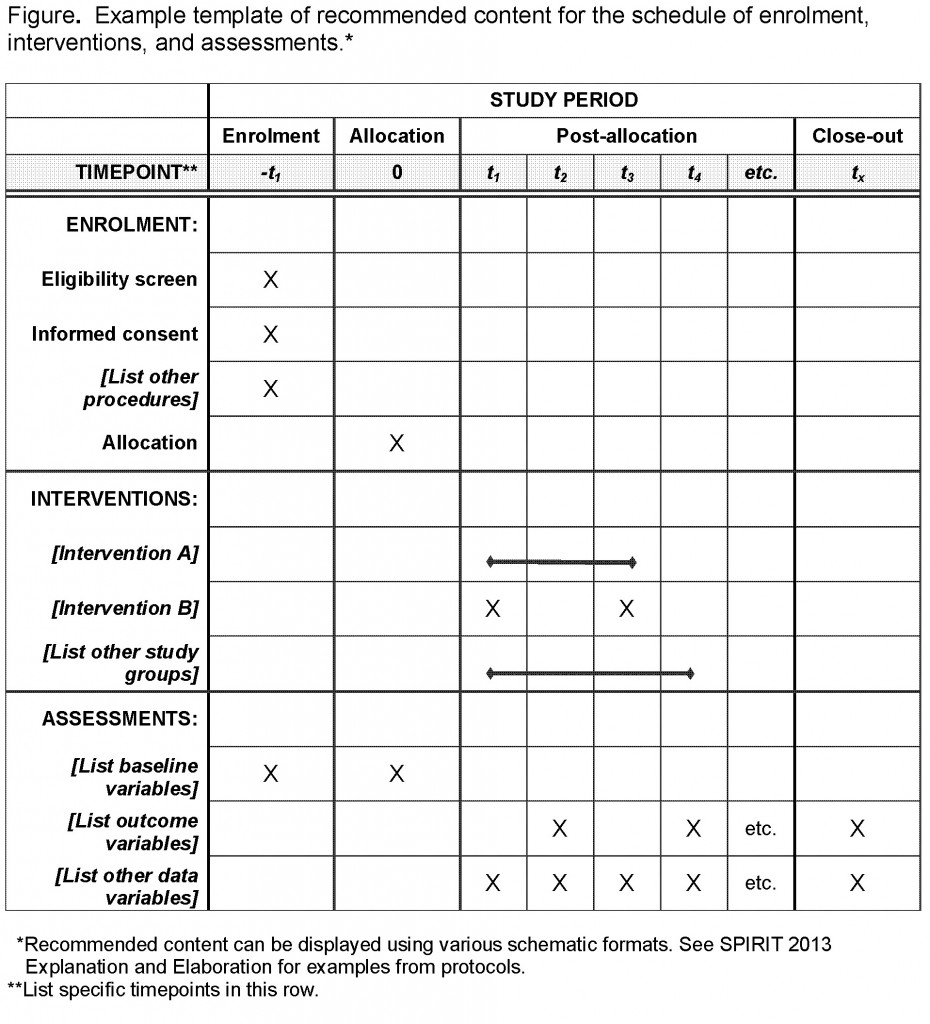
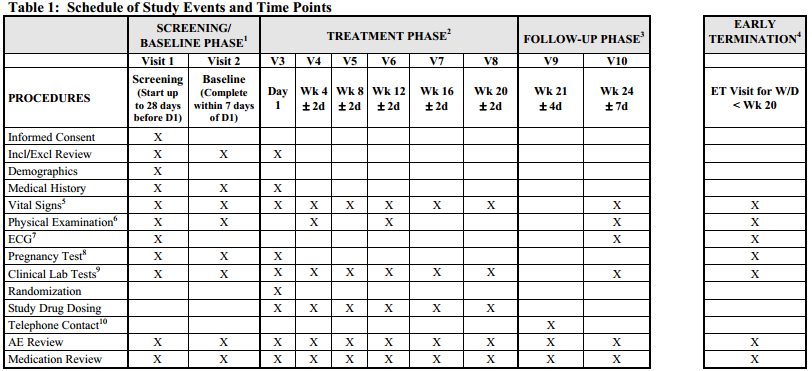
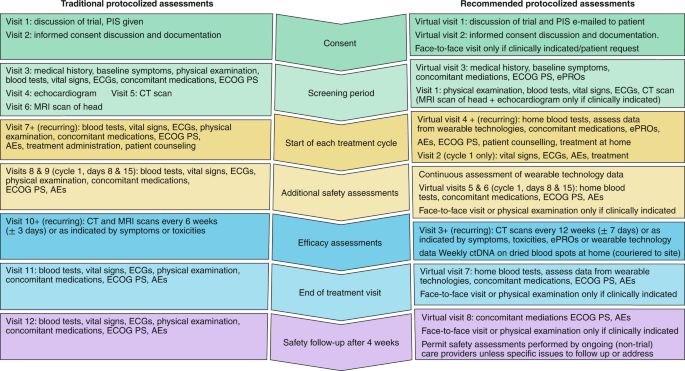
Schedule of Events. Example visit and assessment specification from a... | Download Scientific Diagram

Schedule of Events. Example visit and assessment specification from a... | Download Scientific Diagram

GitHub - RhoInc/participant-visit-listing: an interactive visualization of clinical trial schedule of events by participant
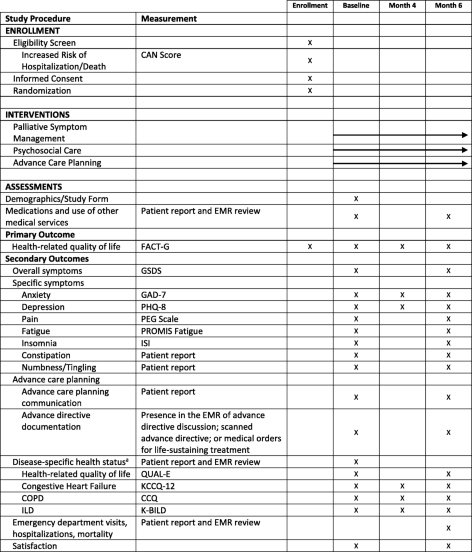
Advancing Symptom Alleviation with Palliative Treatment (ADAPT) trial to improve quality of life: a study protocol for a randomized clinical trial | Trials | Full Text

Table 1 from PCR adjusted cure rates in clinical trials of antimalarial drugs in Africa : Influence of extended follow-up and consecutive day blood sampling | Semantic Scholar