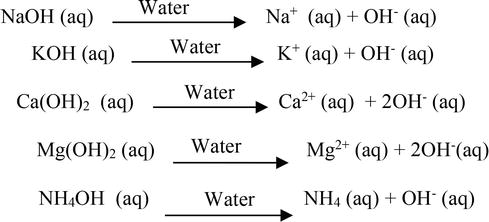
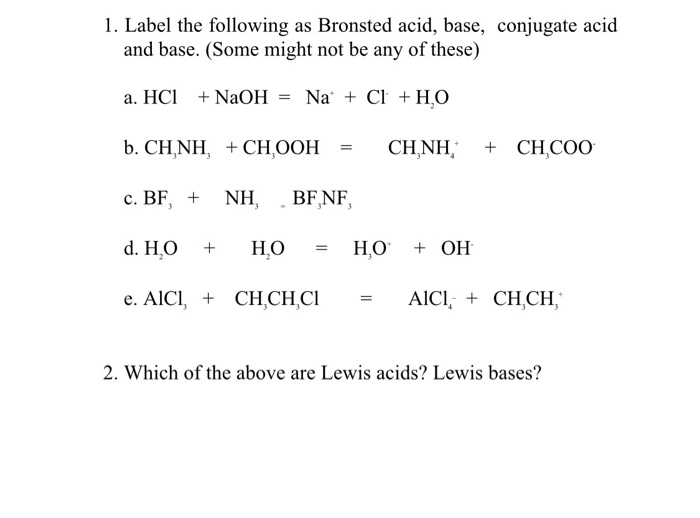
Sodium hydroxide (NaOH) is classified as a strong base. For every mole of sodium hydroxide added to a large volume of water, one mole of what ion enters the solution? | Socratic

RICCA CHEMICAL COMPANY - Sodium Hydroxide is a strong base in terms of chemical ionization and solutions of it can be assayed using a strong acid, such as Hydrochloric Acid or Sulfuric
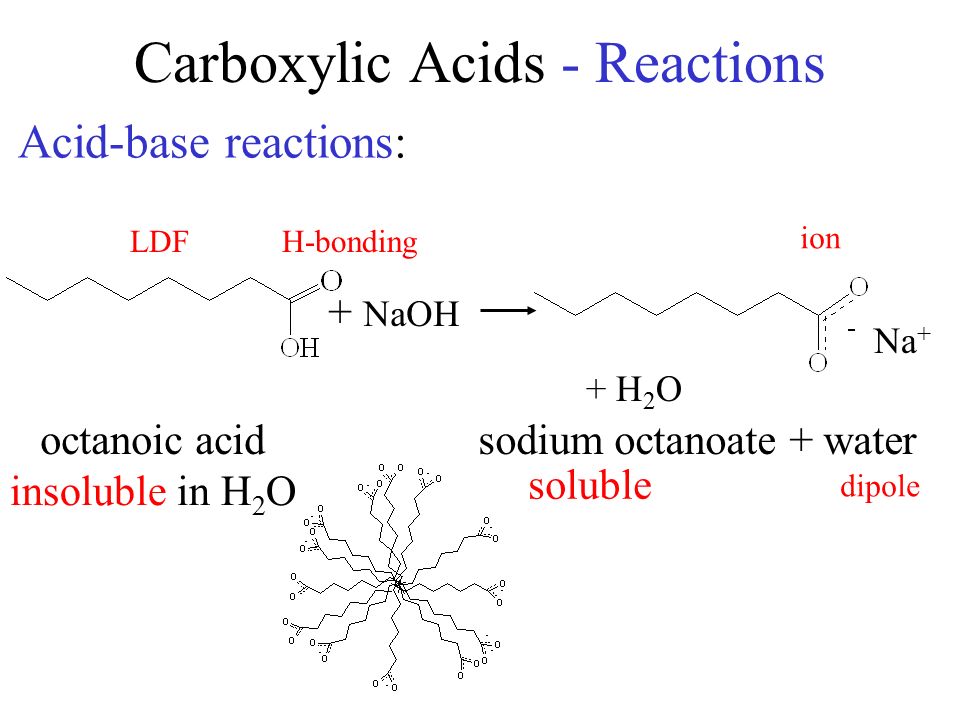
Carboxylic Acids - Reactions Acid-base reactions: octanoic acid + NaOH Na + + H 2 O sodium octanoate + water insoluble in H 2 O soluble H-bondingLDF ion. - ppt download
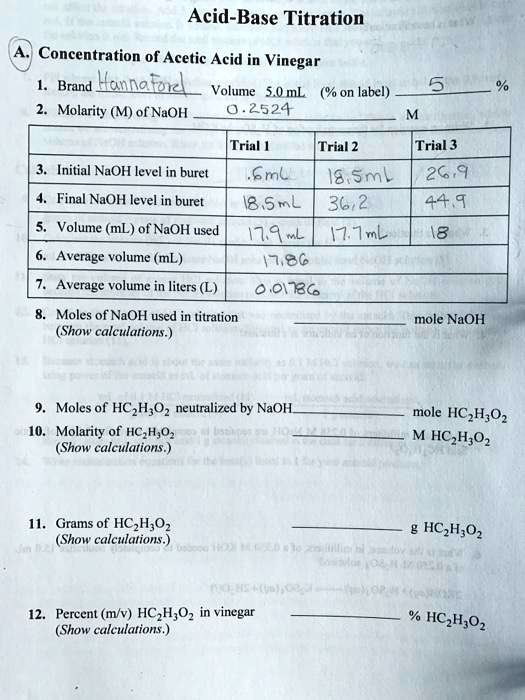
SOLVED: Acid-Base Titration Concentration of Acetic Acid in Vinegar Brand Hanla fud Volume 5.0 mL (% on label) Molarity (M) of NaOH 2524 Trial Trial 2 Trial 3 Initial NaOH level in