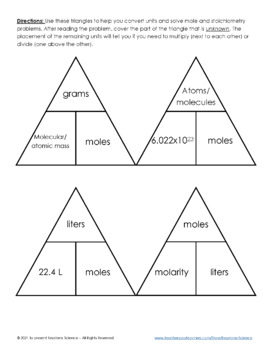
Converting Moles to Grams/Liters/Molecules/Atoms… Do Now: Convert the following What is the conversion factor for a)feet to inches: 1 foot = ___inches. - ppt download

The Moletro Chemistry's Metro. Central Moletro Station 1 “The Mole” Particleville (where atoms and molecules live) Massadonia (where grams live) Liter. - ppt download

Review: Mole Conversions: Convert 3 mols Oxygen to grams: Convert 42 grams Chlorine to mols: What is % composition? What is the %comp of magnesium in magnesium. - ppt video online download


:max_bytes(150000):strip_icc()/606823-calculate-molarity-of-a-solution-FINAL-5b7d7e15c9e77c0050355d4e.png)