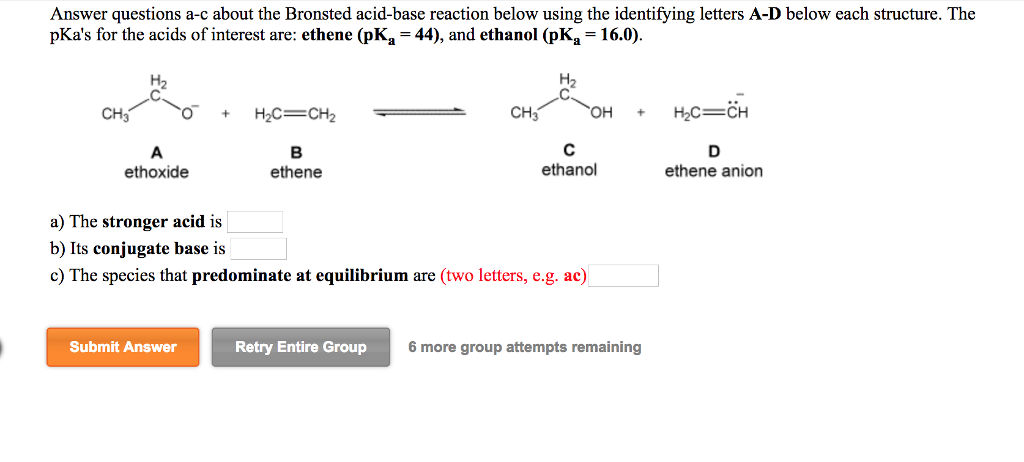
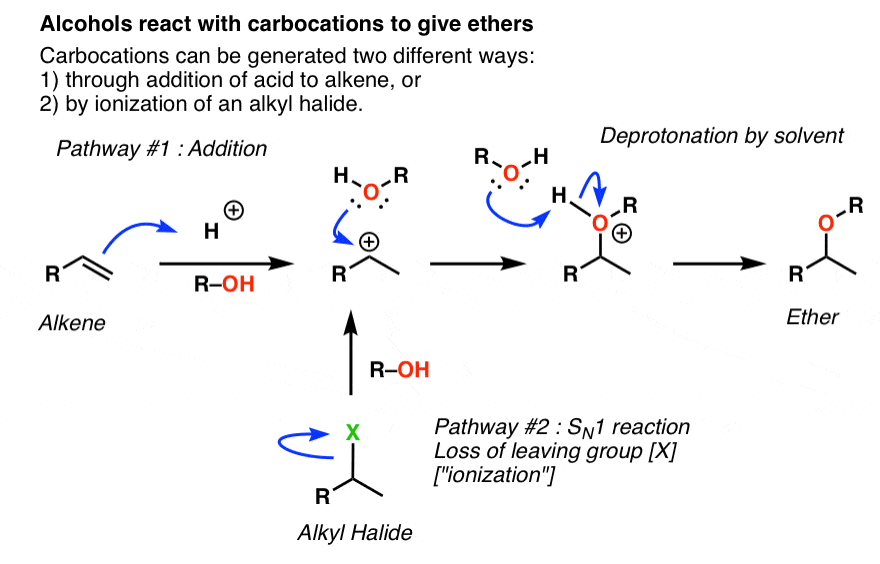
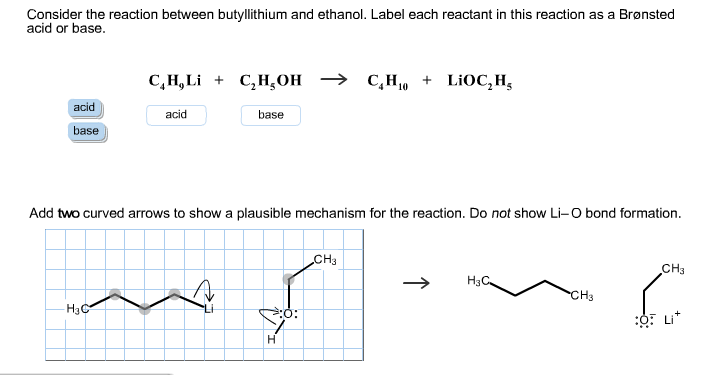
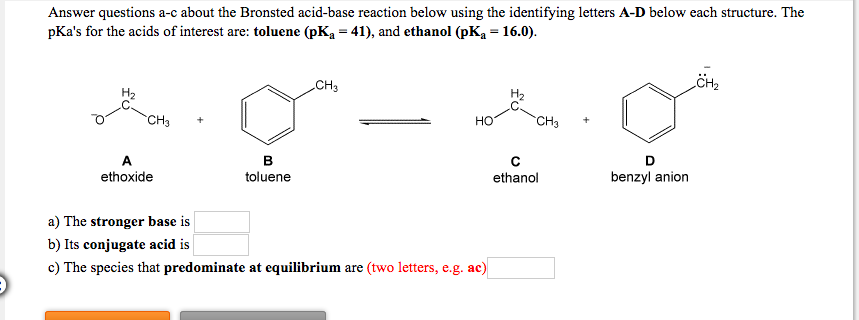
Mechanism and Kinetics of Isobutene Formation from Ethanol and Acetone over ZnxZryOz | ACS Catalysis

Importance of the Nature of the Active Acid/Base Pairs of Hydroxyapatite Involved in the Catalytic Transformation of Ethanol to n‐Butanol Revealed by Operando DRIFTS - Osman - 2019 - ChemCatChem - Wiley Online Library
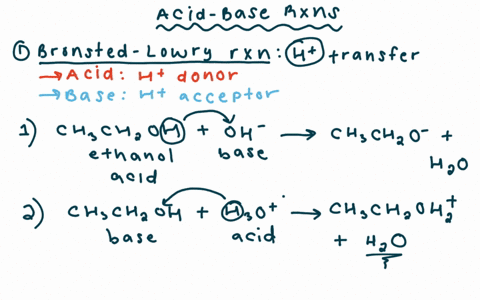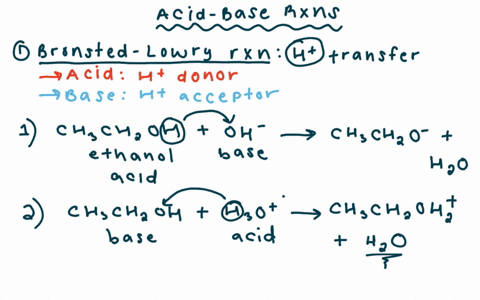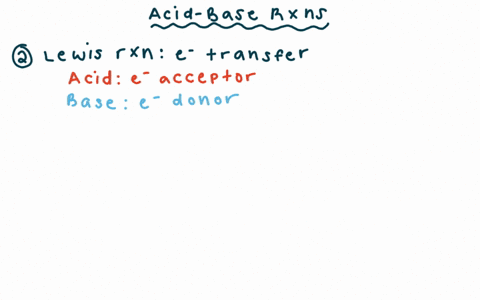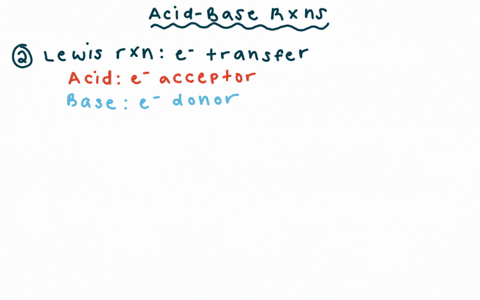
SOLVED:Ethanol (ethyl alcohol), CH3 CH2 OH, can act as a Brønsted-Lowry acid. Write the chemical equation for the reaction of ethanol as an acid with hydroxide ion, OH^-. Ethanol can also react